The overall diagnostic solutions
involve multiple fields,
especially in reproductive health.
Products
Women's Health

The overall diagnostic solutions
involve multiple fields,
especially in reproductive health.
Products
Women's Health
Human Papillomavirus Nucleic Acid Detection and HPV16/18 Genotyping Kit -- HPV 18/2
This kit is a qualitative in vitro test for the detection of human papillomavirus (HPV) in cervical exfoliated epithelial cells specimens. The test not only applies to detect 18 high-risk (HR) HPV types , including type 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, but also can identify the specific types of HPV16 and HPV18 simultaneously.
Methodology: Real-Time PCR, PCR-Fluorescence Probing
Sample Type: Exfoliate Cervical Cell / LBC Specimen
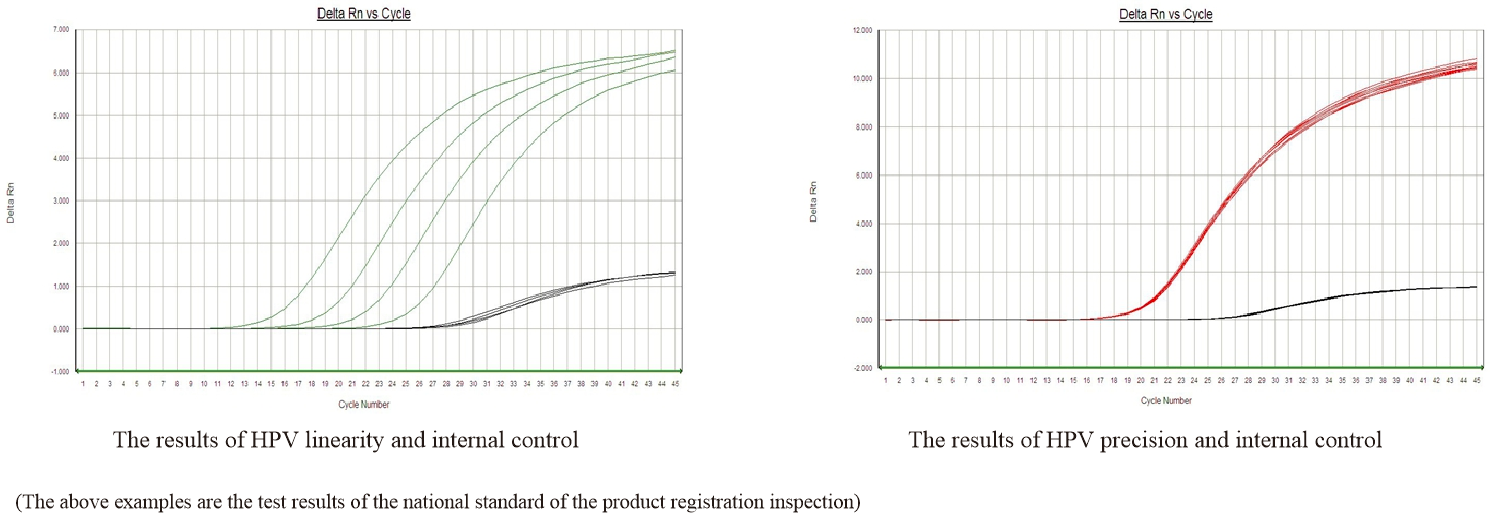
Clinical Sensitivity: 93.2%
Clinical Specificity: 93.0%
Precision: CV <5%
LoD: 5.0 * 104 copies/ml
| 24 tests/kit (non-single test/tube) | 48 tests/kit (non-single test/tube) | 96 tests/lit (non-single test/tube) | 192 tests/lit (non-single test/tube) |
(Specifications can be customized according to actual needs.)